Drawing isomers is a skill we should expect to develop in a Chemistry class. In case you’re not sure what are isomers, they are basically structures that have the same chemical formula (same number of atoms) but are connected differently, therefore, having different structural formula.
The best way to explain how to draw an isomer is to use an example. In this case, we’re going to use C6H14. A tip (Tip #1) that I find useful when drawing isomers is to identify the functional groups present in the given formula. Looking at C6H14, it basically consists of only carbons and hydrogens, making it a hydrocarbon. Now, which type could it be though – is it an alkane, alkene or alkyne? We can figure out which general formula (alkane, alkene or alkyne) C6H14 fits in.
The general formulas of hydrocarbons are:
- alkane: CnH2n+2
- alkene: CnH2n
- alkyne: CnH2n-2
Since we have 6 carbons, that makes n = 6. If we substitute n = 6 into 2n+2, it’ll be 2(6) + 2, and that will give us 14 hydrogens, and what do we know? That’s the same number of hydrogens we have in C6H14! That means C6H14 represents an alkane. Now that we’ve found the functional group for our formula, we can now go to town and start drawing structures with 6 carbons and 14 hydrogens.
The easiest structure to draw is to have 6 carbons connected in a chain, straight in a row like this:

Once we get that done, we can move on to 5 carbons chain, meaning having 5 carbons straight in a row, and then we place the extra 6th carbon on the middle carbons like this:
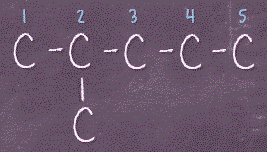

Notice, if we were to stick the 6th carbon on carbon #4 (C4), that’s actually the same as sticking that carbon on C2 (the picture on the left). Now, what about if we were to place that extra carbon on C1 or C5? That will actually give us 6 carbons chain, which is what we have already drawn out at the very beginning. So Tip #2 is, never place the extra carbons (a proper way is to call them substituents) at the end of the chain, as that will only lengthen it. Back to our drawings of 5 carbons chain, looks like we only have 2 ways of them, where the 6th carbon is on either C2 or C3.
Once, we’ve exhausted the possibilities of drawing 5 carbons chain, we can then move on to drawing 4 carbons chain. We’ll draw 4 carbons straight in a row, and then we’ll place the 2 remaining carbons either both on C2 or 1 on C2 and another on C3.
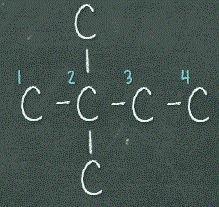

As mentioned in Tip #2, we don’t want to place the extra carbons on either ends, otherwise it’ll give us 5 carbons or 6 carbons chain, which we have already drawn out. With that, we have drawn out the 5 isomers for C6H14.
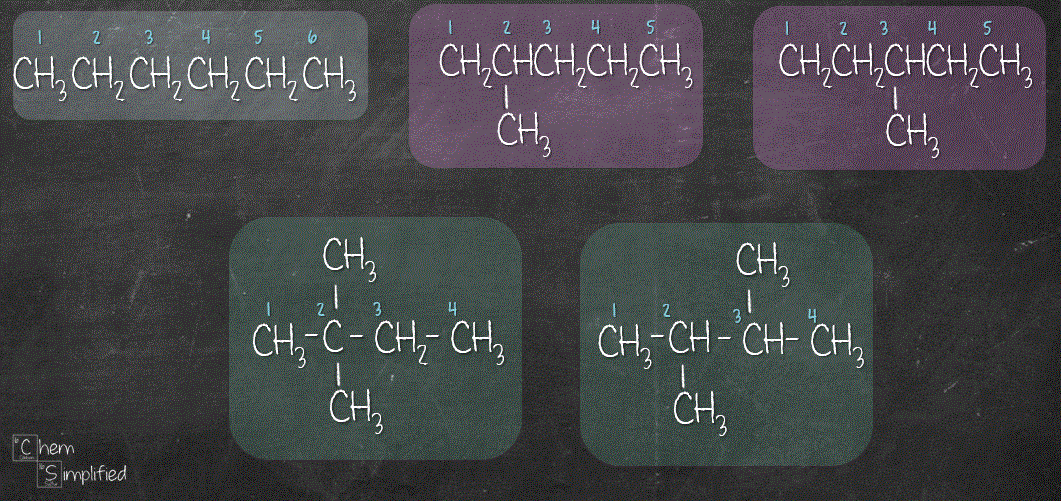
If you’re interested in watching the video where I go through step by step in drawing out all of the isomers for C6H14, definitely check this out:
Want to practise on your own? Challenge your isomer skills to see where you stand. I’ve included some examples that go beyond alkane. We have an assortment of isomers for alcohols, ethers, aldehydes, ketones, carboxylic acids and esters. Oh yea, by the way, here’s Tip #3, if you’ve ever encountered an isomer question that includes oxygen in the formula, you can use this table to quickly figure out some basic isomers you can draw for the given formula (remember the exercise we did at the very beginning to figure out that C6H14 is an alkane?):
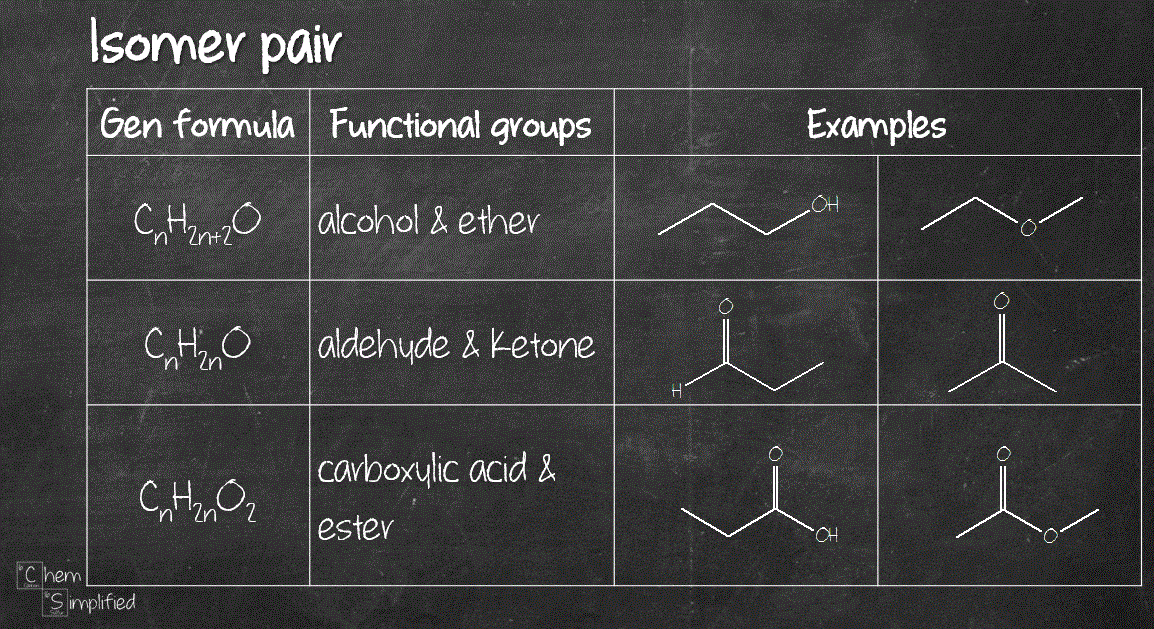
How many isomers are present in C5H12?
How many alcohols and ethers isomers are present in C4H10O?
How many alcohols and ethers isomers are present in C5H12O?
How many aldehydes and ketones isomers are present in C4H8O?
How many aldehydes and ketones isomers are present in C5H10O?
How many carboxylic acids and esters isomers are present in C3H6O2?
How many carboxylic acids and esters isomers are present in C4H8O2?
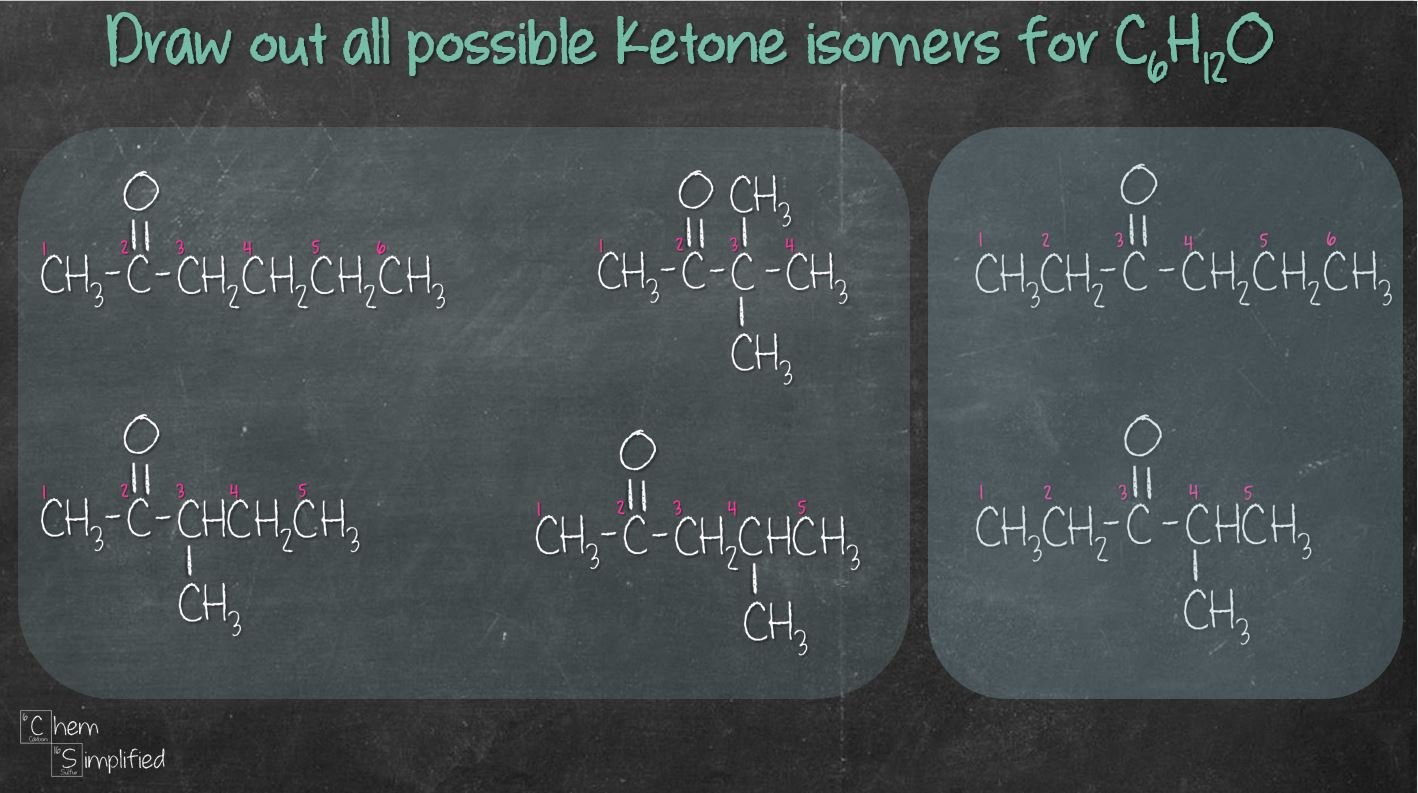
How many ketone isomers are present in C6H12O?

Really helpful. I usually don’t comment on websites, but this one really made me comment. This was extremely helpful, and I have finally grasped the concept of isomers and tysms.
Thanks so much! I’m so glad you find the content helpful and taking the time to leave me a comment. This means a lot to me!
thank you!!! been struggling with these but i think im finally understanding now
Congratulations on your new understanding! And thanks for leaving your comments!
make one for c6h10 please.!
Thanks for your request. I’ll keep that in mind.
It helped me to understand easily. Now, I can draw easily without struggling:
That’s amazing! Congrats.