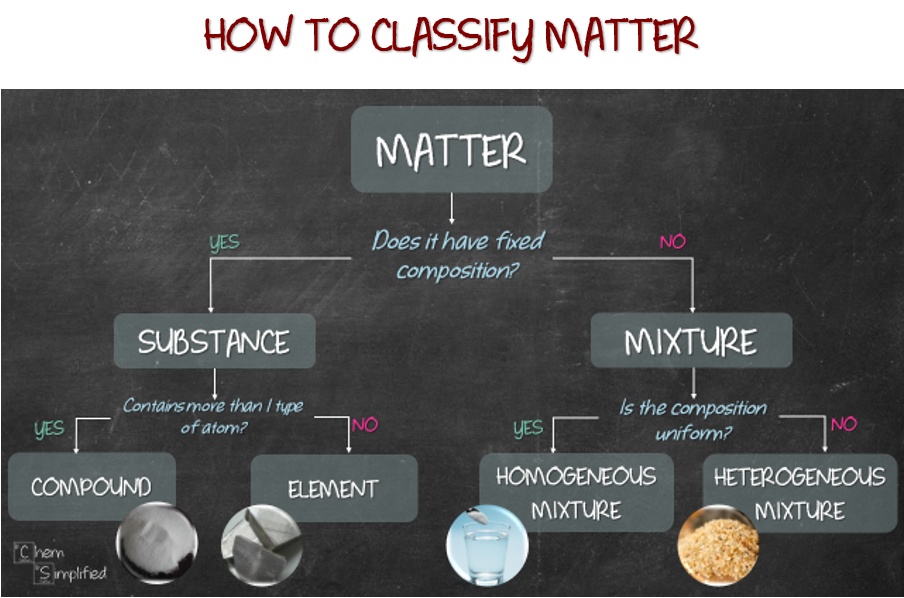
Pure substance and mixture. Element and compound. Homogeneous mixture and heterogeneous mixture. How are they different? Does it MATTER? Yes! WHY? How to classify them? You’re in luck! Ask two questions and you can easily classify matter into element, compound, homogeneous mixture and heterogeneous mixture. Let me show you how.
Let’s classify one of the recipe I’m going to try.
Looks like I’ll be grilling the seasoned chicken on aluminum pan and cover it with aluminum foil. Before that, I’ll need to season it with salt, tumeric powder and other spices.

Let’s start with aluminum foil. Refer to the flow chart at the top for the series of questions and classification. The first question we should always ask is, “Does it have fixed composition?” Do we know what aluminum foil is made out of? Yea, it’s made of aluminum ingot which is flattened to desired thickness. Read more here. Since we know the composition of aluminum foil, which is basically Al, our response to the question is YES. That means it’s a SUBSTANCE. If we need to further classify, then we’ll need to ask the second question, “Does it contain more than 1 type of atom?” Since Al only consists of 1 type of atom, our response will be NO. That means aluminum foil is an ELEMENT.
- Aluminum foil
- Does it have fixed composition?
- Yes >> SUBSTANCE
- Does it contain more than 1 type of atom?
- No >> ELEMENT
- Does it have fixed composition?
Since we’re talking about aluminum foil, let me do a quick side track. Interestingly, aluminum has been linked with diseases such as Alzheimer’s when injected in large quantities into human bodies. Of course, that’s not the same as ingesting small quantities that leach from the foil into your food. Yet if you want to be safe, try to minimize the time the food spends in Aluminium foil, especially when hot.

Alright, back to our classification. Next, let’s classify salt. We know kosher salt is basically table salt without additives. Let’s just assume salt is made out of NaCl and nothing else. So the response to the first question, “Does it have fixed composition?” will be YES, since we know the chemical formula of salt. We know what it is made out of. The response to the next question, “Does it contain more than 1 type of atom?” is YES. This is because NaCl consists of two types of atom, Na and Cl. So, that means salt is a COMPOUND.
- Salt
- Does it have fixed composition?
- Yes >> SUBSTANCE
- Does it contain more than 1 type of atom?
- Yes >> COMPOUND
- Does it have fixed composition?
 Moving on to the other spice needed to season the chicken, tumeric powder. Tumeric powder consists of many components with different percentages, that means it doesn’t have a fixed composition, since not all tumeric powder consist of the exactly the same components. So, answering NO to “Does it have fixed composition?” classifies it as MIXTURE. Then, the next question is, “Is the composition uniform?” Well, if we look at tumeric powder, it’s basically bright dark yellow color throughout. Everything looks basically mixed well. That means, the composition is uniform. Answering YES classified tumeric powder as HOMOGENEOUS MIXTURE.
Moving on to the other spice needed to season the chicken, tumeric powder. Tumeric powder consists of many components with different percentages, that means it doesn’t have a fixed composition, since not all tumeric powder consist of the exactly the same components. So, answering NO to “Does it have fixed composition?” classifies it as MIXTURE. Then, the next question is, “Is the composition uniform?” Well, if we look at tumeric powder, it’s basically bright dark yellow color throughout. Everything looks basically mixed well. That means, the composition is uniform. Answering YES classified tumeric powder as HOMOGENEOUS MIXTURE.
- Tumeric powder
- Does it have fixed composition?
- No >> MIXTURE
- Is the composition uniform?
- Yes >> HOMOGENEOUS MIXTURE
- Does it have fixed composition?

Lastly, the main star of the meal, chicken, specifically boneless chicken thigh. Chicken meat consists of protein, fats, among other things. Therefore, we don’t know its exact composition, therefore the answer to “Does it have fixed composition?” will be NO. Then, to answer the next question, “Is the composition uniform?”, look at the image of chicken thigh. You should be able to see some inconsistent white lines with different shades of reddish pink. This means the composition is not uniform. Therefore, answering NO will classify chicken as HETEROGENEOUS MIXTURE.
- Chicken
- Does it have fixed composition?
- No >> MIXTURE
- Is the composition uniform?
- No >> HETEROGENEOUS MIXTURE
- Does it have fixed composition?
For more examples on how matter is classified, check out the video here:
Wish to practice classifying matter? Each practice set contains 5 questions.


is apple juice a HM
If there is no pulp and it’s a filtered apple juice, then yes, it is a Homogeneous mixture. Apologies on the late reply.
thank you so much! this helped me with my hw in chemistry
I’m so glad! Happy this helps!
What about hard plastic?
It would be a mixture. Depending on how it looks it could be homogeneous mixture (if it’s uniform) or heterogeneous mixture (it looks different at certain parts).