Caution: This method works well for elements in Rows 1 & 2. Elements in row 3 and above may deviate from the guidelines as they can exceed octet.
There’s a general guideline that is helpful in figuring out the number of bonds each element makes. This comes in handy especially when drawing Lewis structures. It’s called the HONC rule, or sometimes known as HONC 1234 rule. The number refers to the number of bonds each of the element makes: Hydrogen makes 1 bond, Oxygen makes 2 bonds, Nitrogen makes 3 bonds and Carbon makes 4 bonds. These four elements are widely used when it comes to drawing Lewis structures at introductory chemistry level.
You might be curious to know why those elements make the stated number of bonds. It’s pretty simple if you look at their Lewis symbols. A quick run through on Lewis symbol. It consists of two parts:
- Chemical symbol
- Valence electrons
Since all elements in the same group share the same pattern in their electron configuration, this means that they have the same number of valence electrons. Therefore it’s easy to group them together, like this:
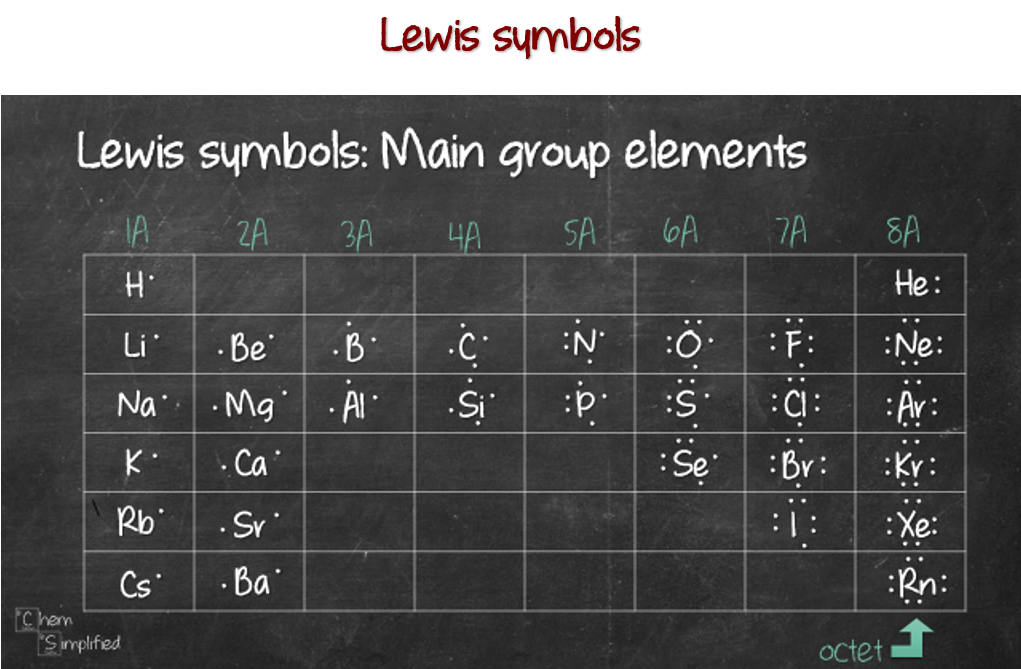
Notice how the electrons are filled when drawing Lewis symbols. Picture 4 imaginary rectangles surrounding the chemical symbol (X), like this:
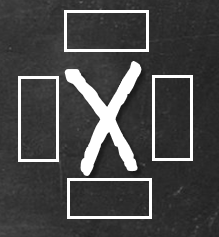
Each of the boxes will take up to 2 electrons maximum, amounting to a total of 8 electrons, representing octet. Once we got the Lewis symbol down, it’s easy to explain why it’s HONC 1234. Let’s start with hydrogen. The Lewis symbol is given on the left. Since there’s room for one more electron (pink box), hydrogen will pair up with 1 electron from another element to form 1 bond.
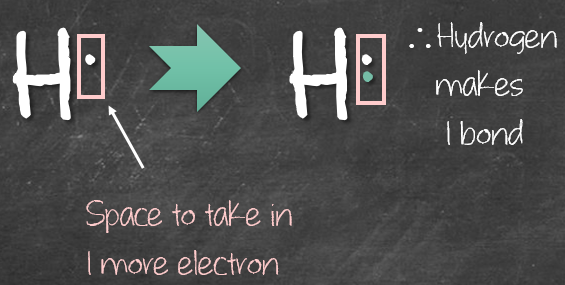
We’ll apply the same reasoning for oxygen. Notice in oxygen, there are already 2 filled boxes (green boxes). Leaving space to take in 2 more electrons in the other 2 (pink) boxes. As a result, oxygen will make 2 bonds.
Using the same explanation for the rest will help explain how elements form the given number of bonds:
- Group 4A elements form 4 bonds
- Group 5A elements form 3 bonds
- Group 6A elements form 2 bonds
- Group 7A elements form 1 bond
Can you explain why Group 3A elements form 3 bonds? Share your thoughts in the comments below.


I think group 3A elements form 3 bonds because they have 3 lone electrons (3 ‘pink boxes’).
You got it! B and Al form 3 bonds when forming covalent compounds, ie. BF3 and AlCl3.
What about tungsten I am kinda stuck on that one.
Tungsten is a tough one because it’s a transition metal, that means, it has variable oxidation number. That also means, it can form multiple number of bonds. That’s why we can safely predict the number of bonds most elements in the main groups (Group 1, 2, 13 – 17) make but not the elements that are in the transition metals block.
How many chemical bonds does ekasilicon form
Bf3 have 4 bond in structure
It’s actually 3. Boron has an exception to octet and is fine with only 6 electrons.
yes, it is correct
How many chemical bonds does ekasilicon form
I suppose it depends on what Germanium is bonded to. Let’s say GeH4, then it would be 4. If it’s GeCl2, then it’s 2.
How many covalent bonds would Ar be able to make?
Ar is a highly stable element, therefore it doesn’t form many types of compound. There are only a few discovered so far and Ar is not bonded the same in all of them. An example is HArF where Ar forms 2 covalent bonds with H and F, respectively.
Question. While studying the organic structure of plants, I found that Sulfur could hold up to 6 bonds. But it shows that it only can create 2 bonds. I’m a little confused
Sulfur indeed can make up to 6 bonds. In fact, it is a multivalent atom, meaning it can make different number of bonds, up to 6, depending on the chemical formula.