Counting atoms … What’s in it for me if I can count atoms correctly?
 You’ll be able to:
You’ll be able to:
- balance chemical equations
- calculate formula/molecular mass
- calculate mols for a specific substance
- perform (terrifying) calculations involving mass-mol-# particles for several substances
Just to be clear, I am talking about counting the number of atoms present in a chemical formula without involving your calculator. Counting the actual number of atoms will come in a later post. In this post, we’ll go through counting atoms from simple to more complex formula. So, are you ready? Feel free to scroll past the easy stuff if you’re already good with the basics.
Here we go …
#1» CH4 [methane]
In this formula, there are two types of atom, carbon (C) and hydrogen (H). You notice the small 4 at the bottom right of hydrogen? That number will tell you how many of that atom is present in the formula. In this case, we have 4 hydrogens. For carbon, notice there’s no small number at its bottom right? That means there’s 1 carbon atom. Only values of 2 and above are written out. If there’s no number written, it means there’s 1. So, in total we have a total of 5 atoms in the CH4, 1 C and 4 H. So here are what we found:
- # C: 1 atom
- #H: 4 atoms
- total # atoms: 5
Doing ok so far? Let’s bring it up a notch.
#2» NH4OH [ammonium hydroxide]
This formula has three types of atoms – nitrogen (N), hydrogen (H) and oxygen (O). Let’s read from left to right. 1 N, 4 H, 1 O and 1 H. Since H appears in two parts in the formula, we should add up the total H atoms: 4 + 1 = 5 H. Why is NH4OH written the way it is? Why not write it as NH5O and make it easier for us to count atoms? You could, but most of the time, you’ll find it’s written as NH4OH so that it’s easier to identify the components that make up this ionic compound – NH4+ and OH–. Don’t sweat it if you don’t get that. I’ll write about it in a future post.
Ok … so back to where we were. Here’s the tally:
- # N: 1 atom
- #H: 5 atoms
- # O: 1 atom
- total # atoms: 7
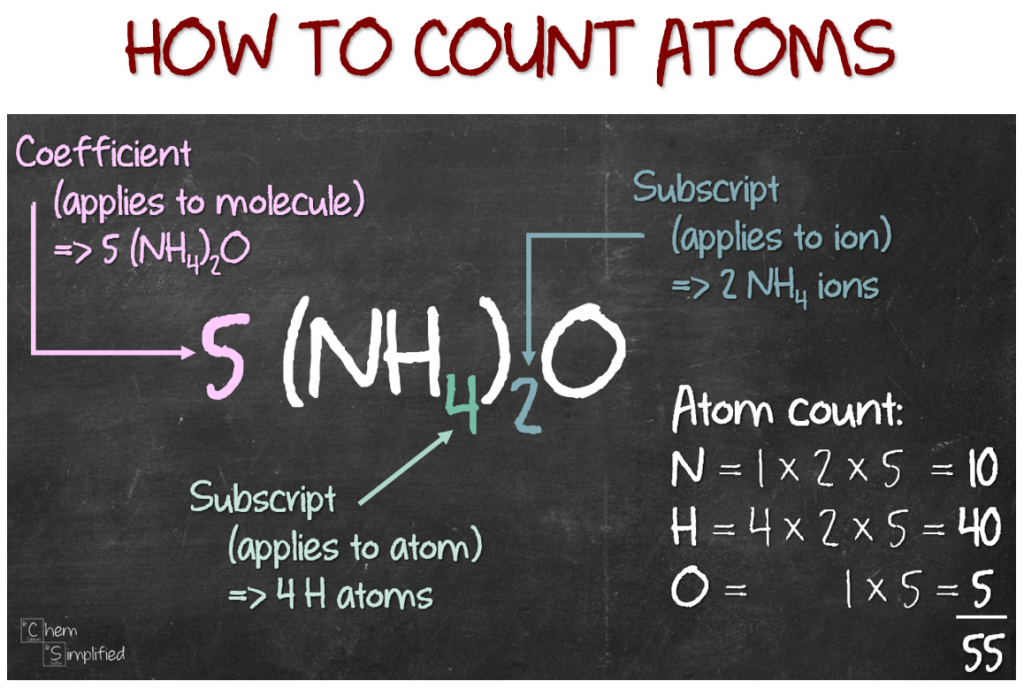
#3» (NH4)2O [ammonium oxide]
This formula looks a bit more complicated than the previous two. What’s up with the bracket? It’s used to easily group a formula together. Notice the bracket covers NH4? And to the bottom right of the bracket, there’s a 2? Well, that means there are 2 groups of NH4. So since there are two groups of NH4, that means we have 2 N in total. From NH4, there’s 1 N, but since there’s a 2 outside of the bracket, that means we actually have 1 × 2 = 2 N. What about H? We apply the same method: 4 × 2 = 8 H. O atom count is a piece of cake by now. Since there’s no number at the bottom right of O, it means we have 1 O. So, here’s what we got:
- # N: 2 atoms
- #H: 8 atoms
- # O: 1 atom
- total # atoms: 11
#4» 3 (NH4)2O
This formula looks quite similar to #3, except there’s a 3 in front of the entire formula. What does that 3 signify? Well, if it’s placed in front of the formula, it means there are 3 of the entire formula. It’s the same as saying (NH4)2O 3 times:(NH4)2O, (NH4)2O, (NH4)2O.
So, if that’s the case, how many N do we have? We have 1 × 2 × 3 = 6 N. How did I get that? Well, inside the bracket, we have 1 N. Then outside the bracket, there’s a 2, that means whatever that’s inside the bracket, we have 2 of it. So that means 1 × 2 (we covered that for example #3 above). But we’re not quite done yet because there’s a 3 in front of the whole formula. So that means whatever we have counted so far for N, there are three times of that (1× 2) + (1× 2) + (1× 2), which is the same as saying 1 × 2 × 3. Doing ok so far?
Let’s continue with H. It’s kind of the same as N. We have 4 × 2 × 3 = 24 H. Inside the bracket, we have 4 H. Then outside the bracket, there’s a 2, that means whatever that’s inside the bracket, we have 2 of it. So that means 4 × 2 (we covered that for example #3 above). Almost there. Remember the 3 in front of the whole formula? So that means whatever we have counted so far for H, there are three times of that, (4× 2) + (4× 2) + (4× 2), which is the same as 4 × 2 × 3. That’s how we got 24 H.
For O, we have 1 × 3 = 3 O. So, here’s what we got:
- # N: 6 atoms
- #H: 24 atoms
- # O: 3 atom
- total # atoms: 33
Let’s bring this up one more notch. What if we have an even more complex formula?
#5» FeC2O4⋅2H2O [iron(II) oxalate dihydrate]
Something new in the formula! There’s a dot right smacked in the middle. What does that mean?? In our example, it means 2 water molecules are trapped in the iron(II) oxalate crystals. That’s a short answer, by the way. If you are curious about the water of crystallization, you can read more about it on Wikipedia. Okay…so what does it mean to us when we are trying to count atoms? Just take it as if 2 H2O is part of the entire molecule. We can count the number atoms before the dot (FeC2O4) in our sleep by now. We have 1 Fe, 2 C and 4 O atoms. But, notice O also appears in H2O after the dot.
So we move on to the new stuff, “⋅2H2O”. Remember what we need to do if there’s a number in front of the term H2O (like in #4 where we have 3 in front of (NH4)2O)? It’s like saying we have 2 of H2O. So how many H do we have? There’s a small 2 at bottom right of H, that means we have 2 H, but since there’s a big 2 in front of H2O, that means we actually have 2 × 2 = 4 H. Apply the same for O count in 2H2O, we have 1 × 2 = 2 O. But since we have some O before the dot, we need to add together the number of O atoms in the formula. It should be 4 + 2 = 6 O. Final tally:
- # Fe: 1 atom
- #C: 2 atoms
- # O: 6 atoms
- # H: 4 atoms
- total # atoms: 13
How are you doing so far? If you’re doing great, let’s continue with something more challenging. If you’re stuck somewhere, how about scrolling up and slowly work your way here?
#6» 5 K3[Fe(C2O4)3]⋅3H2O [potassium iron(III) oxalate trihydrate]
Do you want to try counting atoms in this beast mode formula on your own? Go ahead… I’ll meet you few lines down….(before you start, don’t forget about that 5 in front of the entire formula. Ok…see you few lines down)
.
.
.
.
.
- # K: 15 atoms
- # Fe: 5 atom
- #C: 30 atoms
- # O: 75 atoms
- # H: 30 atoms
- total # atoms: 155
Did you get them all right??? AWESOME, if you did! Here’s how I got mine:
Let’s digest the formula a little. Basically, there are 5 of K3[Fe(C2O4)3]⋅3H2O. Within that formula, we have a dot component, ⋅3H2O. That just means we have 3 H2O molecules within the crystal. In that crystal, we have Fe(C2O4)3. That means we have 1 Fe and 3 groups of C2O4.
Starting with K, there’s 3 of it, but since there’s a 5 in front, the total atoms are 3 × 5 = 15 K.
For Fe, there’s only 1 of it. Multiply with the 5 in front gives us 1 × 5 = 5 Fe.
Next, for C, it’s slightly more complicated. There are 2 C, but since there’s a 3 outside the bracket, it means 2 × 3. And finally, because there’s that 5 in front of the entire molecule, total atoms is 2 × 3 × 5 = 30 C.
O is kind of like C but a little more complicated since it appears in two parts – before the dot and after the dot. Let’s start with before the dot, Fe(C2O4)3: There are 4 O, but since there’s a 3 outside the bracket, it means 4 × 3 = 12. As for the part after the dot, 3H2O: There is 1 O, but since there’s a 3 in front of H2O, we have 1 × 3 = 3. So in total we have 12 + 3 = 15. But since we have a 5 in front of the entire term, we’ll need to multiply by 5, 15 × 5 = 75 O. If you prefer to process it directly, it would be [(4 × 3) + (1 × 3)] × 5 = 75 O. Comes out to the same answer, just depends on how your brain works.
So if we can count O, counting H atom won’t break any sweat. There’s a 2 at the bottom of H, which means we have 2H. But since there’s a 3 in front of H2O, that means it’s 2 × 3. And finally, since there’s a big 5 in front of the entire term, final total H atoms is 2 × 3 × 5 = 30 H.
You are still here? Thanks for sticking around! Hopefully, this post has helped you in one way or another in counting atoms. There are some practice questions for you to build/confirm your confidence. Each click will provide 5 randomized questions. Feel free to boost your confidence as often as you wish.
You might also want to check out the video I posted on this topic:

now I understand with all of the examples you gave me I thank you for making me understand.
now I understand with all of the examples you gave me I thank you for making me understand.
That’s awesome! Congrats on your understanding.
thank you for letting me understand how to count atoms.
You’re most welcome. Glad I could help.
thank you for letting me understand how to count atoms.
You’re most welcome. I’m very glad I could help.
is was amazing now ay understand more thx to dis pages and the persond
That’s really great to know! Congrats on your new understanding!!
Thanks for helpinf me learn this. i was falling behind in class so a little tutoring for myself is always appreciated!
Helping*
It’s really great that you’re paying attention to the fundamentals! This is so crucial before being able to do other stuff like balancing equations, calculating molar mass, etc. You have just saved your future self from wasting time struggling unnecessarily. Congrats!
Thank you for all your help this will definitely help me on future challenges i really appreciate it
You’re most welcome. I’m glad I could help and thanks for taking the time to let me know.
It will help you a lot with your future
hi
Thanks for dropping by!
HOLA
This really helped
Thanks! That’s great to know.